- Record: found
- Abstract: found
- Article: not found
Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung
Abstract
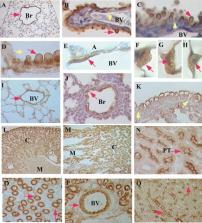
Angiotensin converting enzyme (ACE) 2 is a carboxypeptidase that shares 42% amino acid homology with ACE. Little is known about the regulation or pattern of expression of ACE2 in the mouse lung, including its definitive cellular distribution or developmental changes. Based on Northern blot and RT‐PCR data, we report two distinct transcripts of ACE2 in the mouse lung and kidney and describe a 5′ exon 1a previously unidentified in the mouse. Western blots show multiple isoforms of ACE2, with predominance of a 75–80 kDa protein in the mouse lung versus a 120 kDa form in the mouse kidney. Immunohistochemistry localizes ACE2 protein to Clara cells, type II cells, and endothelium and smooth muscle of small and medium vessels in the mouse lung. ACE2 mRNA levels peak at embryonic day 18.5 in the mouse lung, and immunostaining demonstrates protein primarily in the bronchiolar epithelium at that developmental time point. In murine cell lines ACE2 is strongly expressed in the Clara cell line mtCC, as opposed to the low mRNA expression detected in E10 (type I‐like alveolar epithelial cell line), MLE‐15 (type II alveolar epithelial cell line), MFLM‐4 (fetal pulmonary vasculature cell line), and BUMPT‐7 (renal proximal tubule cell line). In summary, murine pulmonary ACE2 appears to be primarily epithelial, is developmentally regulated, and has two transcripts that include a previously undescribed exon. J. Cell. Biochem. 101:1278–1291, 2007. © 2007 Wiley‐Liss, Inc.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Renal ACE2 expression in human kidney disease.
- Record: found
- Abstract: found
- Article: found
Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus.
- Record: found
- Abstract: found
- Article: not found
