- Record: found
- Abstract: found
- Article: not found
Engineered Nanoscale Lipid-Based Formulation as Potential Enhancer of Gefitinib Lymphatic Delivery: Cytotoxicity and Apoptotic Studies Against the A549 Cell Line

Read this article at
Abstract
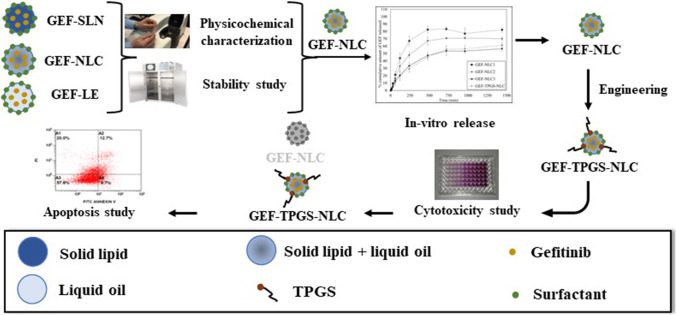
The present study aimed to engineer a nanoscale lipid-based lymphatic drug delivery system with D-α-Tocopherol polyethylene glycol 1000 succinate to combat the lymphatic metastasis of lung cancer. The nanoscale lipid-based systems including GEF-SLN, GEF-NLC, and GEF-LE were prepared and pharmaceutically characterized. In addition, the most stable formulation (GEF-NLC) was subjected to an in vitro release study. Afterward, the optimized GEF-NLC was engineered with TPGS (GEF-TPGS-NLC) and subjected to in vitro cytotoxicity, and apoptotic studies using the A549 cells line as a surrogate model for lung cancer. The present results revealed that particle size and polydispersity index of freshly prepared formulations were ranging from 198 to 280 nm and 0.106 to 0.240, respectively, with negative zeta potential ranging from − 14 to − 27.6.mV. An in vitro release study showed that sustained drug release was attained from GEF-NLC containing a high concentration of lipid. In addition, GEF-NLC and GEF-TPGS-NLC showed remarkable entrapment efficiency above 89% and exhibited sustained release profiles. Cytotoxicity showed that IC 50 of pure GEF was 11.15 μg/ml which decreased to 7.05 μg/ml for GEF-TPGS-NLC. The apoptotic study revealed that GEF-TPGS-NLC significantly decreased the number of living cells from 67 to 58% when compared with pure GEF. The present results revealed that the nanoscale and lipid composition of the fabricated SLN, NLC, and LE could mediate the lymphatic uptake of GEF to combat the lymphatic tumor metastasis. Particularly, GEF-TPGS-NLC is a promising LDDS to increase the therapeutic outcomes of GEF during the treatment of metastatic lung cancer.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells.
- Record: found
- Abstract: found
- Article: not found