- Record: found
- Abstract: found
- Article: found
Systemic administration of the di-apocarotenoid norbixin (BIO201) is neuroprotective, preserves photoreceptor function and inhibits A2E and lipofuscin accumulation in animal models of age-related macular degeneration and Stargardt disease
Read this article at
Abstract
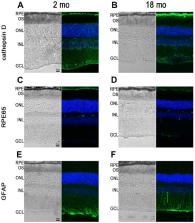
Atrophic A\age-related macular degeneration (AMD) and Stargardt disease (STGD) are major blinding diseases affecting millions of patients worldwide, but no treatment is available. In dry AMD and STGD oxidative stress and subretinal accumulation of N-retinylidene- N-retinylethanolamine (A2E), a toxic by-product of the visual cycle, causes retinal pigment epithelium (RPE) and photoreceptor degeneration leading to visual impairment. Acute and chronic retinal degeneration following blue light damage (BLD) in BALB/c mice and aging of Abca4 -/- Rdh8 -/- mice, respectively, reproduce features of AMD and STGD. Efficacy of systemic administrations of 9'- cis-norbixin (norbixin), a natural di-apocarotenoid, prepared from Bixa orellana seeds with anti-oxidative properties, was evaluated during BLD in BALB/c mice, and in Abca4 -/- Rdh8 -/- mice of different ages, following three experimental designs: “preventive”, “early curative” and “late curative” supplementations. Norbixin injected intraperitoneally in BALB/c mice, maintained scotopic and photopic electroretinogram amplitude and was neuroprotective. Norbixin chronic oral administration for 6 months in Abca4 -/- Rdh8 -/- mice following the “early curative” supplementation showed optimal neuroprotection and maintenance of photoreceptor function and reduced ocular A2E accumulation. Thus, norbixin appears promising as a systemic drug candidate for both AMD and STGD treatment.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes.
- Record: found
- Abstract: found
- Article: not found
Oxidative damage-induced inflammation initiates age-related macular degeneration.
- Record: found
- Abstract: found
- Article: not found