- Record: found
- Abstract: found
- Article: found
The Clinical Significance of Iron Overload and Iron Metabolism in Myelodysplastic Syndrome and Acute Myeloid Leukemia
Read this article at
Abstract
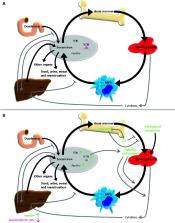
Myelodysplasticsyndrome (MDS) and acute myeloid leukemia (AML) are clonal hematopoietic stem cell diseases leading to an insufficient formation of functional blood cells. Disease-immanent factors as insufficient erythropoiesis and treatment-related factors as recurrent treatment with red blood cell transfusions frequently lead to systemic iron overload in MDS and AML patients. In addition, alterations of function and expression of proteins associated with iron metabolism are increasingly recognized to be pathogenetic factors and potential vulnerabilities of these diseases. Iron is known to be involved in multiple intracellular and extracellular processes. It is essential for cell metabolism as well as for cell proliferation and closely linked to the formation of reactive oxygen species. Therefore, iron can influence the course of clonal myeloid disorders, the leukemic environment and the occurrence as well as the defense of infections. Imbalances of iron homeostasis may induce cell death of normal but also of malignant cells. New potential treatment strategies utilizing the importance of the iron homeostasis include iron chelation, modulation of proteins involved in iron metabolism, induction of leukemic cell death via ferroptosis and exploitation of iron proteins for the delivery of antileukemic drugs. Here, we provide an overview of some of the latest findings about the function, the prognostic impact and potential treatment strategies of iron in patients with MDS and AML.
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease

- Record: found
- Abstract: found
- Article: found