- Record: found
- Abstract: found
- Article: found
Correction: GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function
correction
Anmol Kumar ,
Jaakko Kopra ,
Kärt Varendi ,
Lauriina L. Porokuokka ,
Anne Panhelainen ,
Satu Kuure ,
Pepin Marshall ,
Nina Karalija ,
Mari-Anne Härma ,
Carolina Vilenius ,
Kersti Lilleväli ,
Triin Tekko ,
Jelena Mijatovic ,
Nita Pulkkinen ,
Madis Jakobson ,
Maili Jakobson ,
Roxana Ola ,
Erik Palm ,
Maria Lindahl ,
Ingrid Strömberg ,
Vootele Võikar ,
T. Petteri Piepponen ,
Mart Saarma ,
Jaan-Olle Andressoo
11 January 2016
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The y-axis values in Fig 4M are incorrect. Please view the correct figure here.
10.1371/journal.pgen.1005808.g001
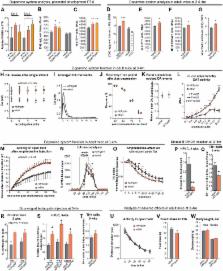
Fig 4
Increased endogenous GDNF expression affects the development and function of the nigrostriatal
dopaminergic system.
(A) Levels of phosphorylated ERK2 at P7.5 in the striatum of Gdnf
wt/wt
, Gdnf
wt/hyper
and Gdnf
hyper/hyper
mice. N = 5 mice/group; ERK was used for normalization. (B) HPLC analysis of DA levels
in the rostral brain; N = 5–8 mice/group (F = 7.44, P = 0.016). (C) Quantification
of tyrosine hydroxylase (TH)-positive (a marker of DA neurons) cells in the SNpc;
N = 6–8 mice/group (F = 7.44, P = 0.0048). (D) HPLC analysis of DA levels in the dSTR;
N = 11 for Gdnf
wt/wt
, 8 for Gdnf
wt/hyper
mice/group (P = 0.000164). HPLC analysis of DA levels in the dorsal striatum of Gdnf
3’UTR
wt/wt
and Gdnf
wt/KO
mice; N = 6 mice/group. (E-F) The number of TH-positive (E; N = 8 Gdnf
wt/wt, N = 7 Gdnf
wt/hyper; P = 0.025) and VMAT2-positive neurons (F; N = 7 Gdnf
wt/wt, N = 7 Gdnf
wt/hyper; P = 0.016) in the SNpc. (G) The number of DAT+ varicosities (N = 9 Gdnf
wt/wt, N = 7 Gdnf
wt/hyper; P = 0.042) in the dSTR. (H-K) Cyclic voltammetry analysis of acute striatal
slices (see also S3J Fig); N = 5–7 mice/group with 1–3 slices per mouse. (H) DA release
in response to electrical stimulation [two-way repeated measures ANOVA, F (1,29) =
5.866]; (I) Averaged traces of DA events. (J) Short-term depression of striatal DA
release after prior DA exocytosis, shown as percent of the first DA release. (K) The
ratio of DA release after a single stimulus and after a 5 pulse burst at 20Hz. (L)
In vivo amperometry following intrastriatal DA injection reveals that dopamine transporter
(DAT) activity in Gdnf
wt/hyper
mice is dependent on the concentration of DA; N = 4 mice/group (F = 47.931). (M) Locomotor
activity after an injection of amphetamine (1 mg/kg, i.p.); N = 9–10 mice/group (F
= 4.386, P = 0.04). (N) In vivo microdialysis analysis of extracellular striatal DA
levels; amphetamine was applied as indicated by the horizontal bar; N = 9 mice/group.
(O) Cyclic voltammetry analysis shows that amphetamine (5 μM) depletes stimulated
DA release faster in the striata of Gdnf
wt/hyper
mice compared to Gdnf
wt/wt
mice; two-way repeated-measures ANOVA reveals an effect of time (P<0.0001) and genotype
(P = 0.031), as well as an interaction between time and genotype (P = 0.049); N =
6 mice/group with 1–3 slices per mouse. (P-Q) Analysis of a 6-OHDA induced PD model.
(P) Quantification of DA in the dSTR 2 weeks after striatal 6-OHDA injection, relative
to the intact side (N = 12 Gdnf
wt/wt; N = 10 Gdnf
wt/hyper), (F = 40.62, P = 0.00549, Students t-test). The intact and lesioned side
differed significantly (P = 2.71×10−15). (Q) Quantification of TH-positive neurons
in the SNpc 2 weeks after striatal 6-OHDA injection, relative to the intact side,
(F = 7.04, P = 0.0143, Students t-test). The intact and lesioned side differed significantly
(P = 3.00×10−11). (R-T) Analysis of a lactacystin-induced PD model. (R) The percentage
of sugar pellet retrievals from the contralateral side in the corridor test; N = 5–7
mice/group (F = 6.087, P = 0.033). (S) Quantification of DA, DOPAC, and HVA in the
dSTR 5 weeks after supranigral lactacystin injection, relative to the intact side;
N = 5 Gdnf
wt/wt
, N = 7 Gdnf
wt/hyper
; P = 0.046 for DA, P = 0.015 for DOPAC, P = 0.011 for HVA. The intact and lesioned
side differed significantly; P = 0.00016 for DA, P = 0.015 for DOPAC, P = 0.010 for
HVA. (T) Quantification of TH-positive neurons in the SNpc 5 weeks after lactacystin
injection, relative to the intact side; N = 4 Gdnf
wt/wt
, N = 7 Gdnf
wt/hyper
; P = 0.236. The intact and lesioned side differed significantly (P = 0.00029). (U-W)
Evaluation of side effects associated with intracranial ectopic GDNF expression. (U)
Spontaneous locomotor activity in an open field; N = 31–34 mice/group. (V) Food intake
by adult mice during a 72-hour period; N = 10 mice/group. (W) Body weight of adult
mice; N = 9–34 mice/group. Abbreviations: DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic
acid; HVA, homovanillic acid; dSTR, dorsal striatum; SNpc, substantia nigra pars compacta.
Related collections
Most cited references1

- Record: found
- Abstract: found
- Article: found
GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function
Anmol Kumar, Jaakko Kopra, Kärt Varendi … (2015)