- Record: found
- Abstract: found
- Article: found
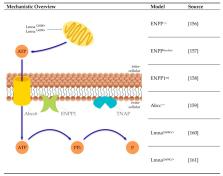
Research Models for Studying Vascular Calcification
Read this article at
Abstract
Calcification of the vessel wall contributes to high cardiovascular morbidity and mortality. Vascular calcification (VC) is a systemic disease with multifaceted contributing and inhibiting factors in an actively regulated process. The exact underlying mechanisms are not fully elucidated and reliable treatment options are lacking. Due to the complex pathophysiology, various research models exist evaluating different aspects of VC. This review aims to give an overview of the cell and animal models used so far to study the molecular processes of VC. Here, in vitro cell culture models of different origins, ex vivo settings using aortic tissue and various in vivo disease-induced animal models are summarized. They reflect different aspects and depict the (patho)physiologic mechanisms within the VC process.
Related collections
Most cited references153
- Record: found
- Abstract: found
- Article: not found
Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro.
- Record: found
- Abstract: not found
- Article: not found
Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism
- Record: found
- Abstract: found
- Article: not found