- Record: found
- Abstract: found
- Article: found
Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K
Read this article at
Abstract
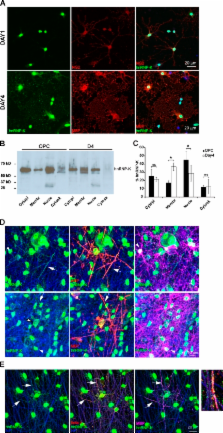
α6β1-integrin interacts with hnRNP-K, an mRNA-binding protein, during oligodendrocyte differentiation to promote translation of MBP mRNA and myelin synthesis.
Abstract
Myelination in the central nervous system provides a unique example of how cells establish asymmetry. The myelinating cell, the oligodendrocyte, extends processes to and wraps multiple axons of different diameter, keeping the number of wraps proportional to the axon diameter. Local regulation of protein synthesis represents one mechanism used to control the different requirements for myelin sheath at each axo–glia interaction. Prior work has established that β1-integrins are involved in the axoglial interactions that initiate myelination. Here, we show that integrin activation regulates translation of a key sheath protein, myelin basic protein (MBP), by reversing the inhibitory effect of the mRNA 3′UTR. During oligodendrocyte differentiation and myelination α6β1-integrin interacts with hnRNP-K, an mRNA-binding protein, which binds to MBP mRNA and translocates from the nucleus to the myelin sheath. Furthermore, knockdown of hnRNP-K inhibits MBP protein synthesis during myelination. Together, these results identify a novel pathway by which axoglial adhesion molecules coordinate MBP synthesis with myelin sheath formation.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta.
- Record: found
- Abstract: found
- Article: not found
Myelin basic protein: a multifunctional protein.
- Record: found
- Abstract: found
- Article: not found