- Record: found
- Abstract: found
- Article: not found
Translational regulation of Inhibin βA by TGFβ via the RNA-binding protein hnRNP E1 enhances the invasiveness of epithelial-to-mesenchymal transitioned cells
Abstract
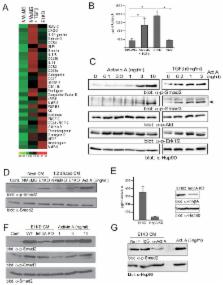
The epithelial-to-mesenchymal transition (EMT) is a cellular process that functions during embryonic development and tissue regeneration, thought to be aberrantly activated in epithelial-derived cancer and play an important role in the process of metastasis. The TGFβ signaling pathway is a key inducer of EMT and we have elucidated a post-transcriptional mechanism by which TGFβ modulates expression of select transcripts via the RNA binding protein hnRNP E1 during EMT. One such transcript inhibin βA is a member of the TGFβ superfamily. Here, we show by polysome profiling that inhibin βA is translationally regulated by TGFβ via hnRNP E1. TGFβ treatment or knockdown of hnRNP E1 relieves silencing of the inhibin βA transcript, resulting in increased protein expression and secreted levels of the inhibin βA homodimer, activin A. Our data indicates that the translational up-regulation of inhibin βA enhances the migration and invasion of cells that have undergone an EMT and promotes cancer progression in vivo.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found
TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility.
- Record: found
- Abstract: found
- Article: not found
