- Record: found
- Abstract: found
- Article: found
Nuclear F-actin and Lamin A antagonistically modulate nuclear shape
Read this article at
ABSTRACT
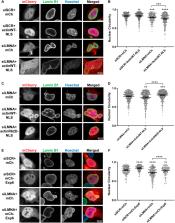
Nuclear shape influences cell migration, gene expression and cell cycle progression, and is altered in disease states like laminopathies and cancer. What factors and forces determine nuclear shape? We find that nuclei assembled in Xenopus egg extracts in the presence of dynamic F-actin exhibit a striking bilobed nuclear morphology with distinct membrane compositions in the two lobes and accumulation of F-actin at the inner nuclear envelope. The addition of Lamin A (encoded by lmna), which is absent from Xenopus eggs, results in rounder nuclei, suggesting that opposing nuclear F-actin and Lamin A forces contribute to the regulation of nuclear shape. Nuclear F-actin also promotes altered nuclear shape in Lamin A-knockdown HeLa cells and, in both systems, abnormal nuclear shape is driven by formins and not Arp2/3 or myosin. Although the underlying mechanisms might differ in Xenopus and HeLa cells, we propose that nuclear F-actin filaments nucleated by formins impart outward forces that lead to altered nuclear morphology unless Lamin A is present. Targeting nuclear actin dynamics might represent a novel approach to rescuing disease-associated defects in nuclear shape.
Abstract
Summary: Formin-nucleated F-actin and Lamin A antagonistically modulate nuclear shape in Xenopus egg extracts and HeLa cells, which might be relevant in diseases with altered nuclear morphology.
Related collections
Most cited references94
- Record: found
- Abstract: found
- Article: not found
Nuclear envelope rupture and repair during cancer cell migration.
- Record: found
- Abstract: found
- Article: not found
A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage.
- Record: found
- Abstract: found
- Article: not found