- Record: found
- Abstract: found
- Article: found
GTSE1 regulates spindle microtubule dynamics to control Aurora B kinase and Kif4A chromokinesin on chromosome arms
Read this article at
Abstract
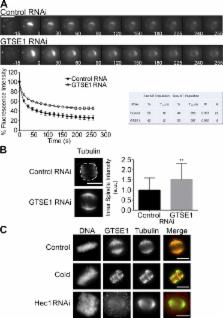
Mitosis involves complex interactions of multiple pathways, some yet uncharacterized. Tipton et al. use bioinformatics to implicate GTSE1 in mitosis. Depletion of GTSE1 disrupts chromosome alignment and spindle pole integrity, in part because of hyperstabilization of spindle microtubules and depletion of Aurora B kinase and Kif4A chromokinesin on chromosome arms.
Abstract
In mitosis, the dynamic assembly and disassembly of microtubules are critical for normal chromosome movement and segregation. Microtubule turnover varies among different mitotic spindle microtubules, dictated by their spatial distribution within the spindle. How turnover among the various classes of spindle microtubules is differentially regulated and the resulting significance of differential turnover for chromosome movement remains a mystery. As a new tactic, we used global microarray meta-analysis (GAMMA), a bioinformatic method, to identify novel regulators of mitosis, and in this study, we describe G2- and S phase–expressed protein 1 (GTSE1). GTSE1 is expressed exclusively in late G2 and M phase. From nuclear envelope breakdown until anaphase onset, GTSE1 binds preferentially to the most stable mitotic spindle microtubules and promotes their turnover. Cells depleted of GTSE1 show defects in chromosome alignment at the metaphase plate and in spindle pole integrity. These defects are coupled with an increase in the proportion of stable mitotic spindle microtubules. A consequence of this reduced microtubule turnover is diminished recruitment and activity of Aurora B kinase on chromosome arms. This decrease in Aurora B results in diminished binding of the chromokinesin Kif4A to chromosome arms.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
The spindle-assembly checkpoint in space and time.
- Record: found
- Abstract: found
- Article: not found
Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores
- Record: found
- Abstract: found
- Article: not found