- Record: found
- Abstract: found
- Article: found
The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism
Read this article at
Abstract
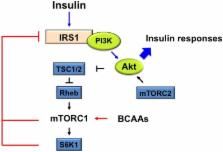
Insulin is required for maintenance of glucose homeostasis. Despite the importance of insulin sensitivity to metabolic health, the mechanisms that induce insulin resistance remain unclear. Branched-chain amino acids (BCAAs) belong to the essential amino acids, which are both direct and indirect nutrient signals. Even though BCAAs have been reported to improve metabolic health, an increased BCAA plasma level is associated with a high risk of metabolic disorder and future insulin resistance, or type 2 diabetes mellitus (T2DM). The activation of mammalian target of rapamycin complex 1 (mTORC1) by BCAAs has been suggested to cause insulin resistance. In addition, defective BCAA oxidative metabolism might occur in obesity, leading to a further accumulation of BCAAs and toxic intermediates. This review provides the current understanding of the mechanism of BCAA-induced mTORC1 activation, as well as the effect of mTOR activation on metabolic health in terms of insulin sensitivity. Furthermore, the effects of impaired BCAA metabolism will be discussed in detail.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Sestrin2 is a leucine sensor for the mTORC1 pathway.
- Record: found
- Abstract: found
- Article: not found
Growing roles for the mTOR pathway.
- Record: found
- Abstract: found
- Article: not found