- Record: found
- Abstract: found
- Article: found
Kinesin-5 inhibitor resistance is driven by kinesin-12
Read this article at
Abstract
The kinesin-5 Eg5 is essential for mitotic progression, and the lethal effects of Eg5 inhibitors make these inhibitors attractive candidates for chemotherapy drugs. Sturgill et al. show that kinesin-12 and a nonmotile Eg5 mutant form an alternative spindle assembly pathway that provides resistance to Eg5 inhibitors.
Abstract
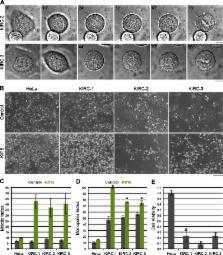
The microtubule (MT) cytoskeleton bipolarizes at the onset of mitosis to form the spindle. In animal cells, the kinesin-5 Eg5 primarily drives this reorganization by actively sliding MTs apart. Its primacy during spindle assembly renders Eg5 essential for mitotic progression, demonstrated by the lethal effects of kinesin-5/Eg5 inhibitors (K5Is) administered in cell culture. However, cultured cells can acquire resistance to K5Is, indicative of alternative spindle assembly mechanisms and/or pharmacological failure. Through characterization of novel K5I-resistant cell lines, we unveil an Eg5 motility-independent spindle assembly pathway that involves both an Eg5 rigor mutant and the kinesin-12 Kif15. This pathway centers on spindle MT bundling instead of Kif15 overexpression, distinguishing it from those previously described. We further show that large populations (∼10 7 cells) of HeLa cells require Kif15 to survive K5I treatment. Overall, this study provides insight into the functional plasticity of mitotic kinesins during spindle assembly and has important implications for the development of antimitotic regimens that target this process.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes.
- Record: found
- Abstract: found
- Article: not found
Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1.
- Record: found
- Abstract: found
- Article: not found