- Record: found
- Abstract: found
- Article: found
Induction of IL-17A Precedes Development of Airway Hyperresponsiveness during Diet-Induced Obesity and Correlates with Complement Factor D
Read this article at
Abstract
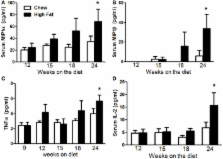
Obesity is a risk factor for the development of asthma. Obese mice exhibit innate airway hyperresponsiveness (AHR), a characteristic feature of asthma, and IL-17A is required for development of AHR in obese mice. The purpose of this study was to examine the temporal association between the onset of AHR and changes in IL-17A during the development of obesity by high-fat feeding in mice. At weaning, C57BL/6J mice were placed either on mouse chow or on a high-fat diet (HFD) and examined 9, 12, 15, 18, or 24 weeks later. Airway responsiveness to aerosolized methacholine (assessed via the forced oscillation technique) was greater in mice fed HFD versus chow for 24 weeks but not at earlier time points. Bronchoalveolar lavage and serum IL-17A were not affected by either the type or duration of diet, but increased pulmonary IL17a mRNA abundance was observed in HFD versus chow fed mice after both 18 and 24 weeks. Flow cytometry also confirmed an increase in IL-17A + γδ T cells and IL-17A + CD4 + T (Th17) cells in lungs of HFD versus chow fed mice. Pulmonary expression of Cfd (complement factor D, adipsin), a gene whose expression can be reduced by IL-17A, decreased after both 18 and 24 weeks in HFD versus chow fed mice. Furthermore, pulmonary Cfd mRNA abundance correlated with elevations in pulmonary Il17a mRNA expression and with AHR. Serum levels of TNFα, MIP-1α, and MIP-1β, and classical markers of systemic inflammation of obesity were significantly greater in HFD than chow fed mice after 24 weeks, but not earlier. In conclusion, our data indicate that pulmonary rather than systemic IL-17A is important for obesity-related AHR and suggest that changes in pulmonary Cfd expression contribute to these effects of IL-17A. Further, the observation that increases in Il17a preceded the development of AHR by several weeks suggests that IL-17A interacts with other factors to promote AHR. The observation that the onset of the systemic inflammation of obesity coincided temporally with the development of AHR suggest that systemic inflammation may be one of these factors.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model
- Record: found
- Abstract: found
- Article: not found
Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies.
- Record: found
- Abstract: found
- Article: not found