- Record: found
- Abstract: found
- Article: found
Reversing Autoimmunity Combination of Rituximab and Intravenous Immunoglobulin
Read this article at
Abstract
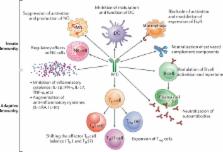
In this concept paper, the authors present a unique and novel protocol to treat autoimmune diseases that may have the potential to reverse autoimmunity. It uses a combination of B cell depletion therapy (BDT), specifically rituximab (RTX) and intravenous immunoglobulin (IVIg), based on a specifically designed protocol (Ahmed Protocol). Twelve infusions of RTX are given in 6–14 months. Once the CD20 + B cells are depleted from the peripheral blood, IVIg is given monthly until B cells repopulation occurs. Six additional cycles are given to end the protocol. During the stages of B cell depletion, repopulation and after clinical recovery, IVIg is continued. Along with clinical recovery, significant reduction and eventual disappearance of pathogenic autoantibody occurs. Administration of IVIg in the post-clinical period is a crucial part of this protocol. This combination reduces and may eventually significantly eliminates inflammation in the microenvironment and facilitates restoring immune balance. Consequently, the process of autoimmunity and the phenomenon that lead to autoimmune disease are arrested, and a sustained and prolonged disease and drug-free remission is achieved. Data from seven published studies, in which this combination protocol was used, are presented. It is known that BDT does not affect check points. IVIg has functions that mimic checkpoints. Hence, when inflammation is reduced and the microenvironment is favorable, IVIg may restore tolerance. The authors provide relevant information, molecular mechanism of action of BDT, IVIg, autoimmunity, and autoimmune diseases. The focus of the manuscript is providing an explanation, using the current literature, to demonstrate possible pathways, used by the combination of BDT and IVIg in providing sustained, long-term, drug-free remissions of autoimmune diseases, and thus reversing autoimmunity, albeit for the duration of the observation.
Related collections
Most cited references137
- Record: found
- Abstract: found
- Article: not found
Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire.
- Record: found
- Abstract: found
- Article: found
Human regulatory B cells in health and disease: therapeutic potential
- Record: found
- Abstract: found
- Article: not found