- Record: found
- Abstract: found
- Article: found
Transition from inflammation to proliferation: a critical step during wound healing
Read this article at
Abstract
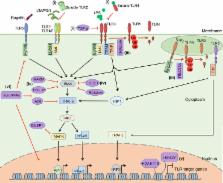
The ability to rapidly restore the integrity of a broken skin barrier is critical and is the ultimate goal of therapies for hard-to-heal-ulcers. Unfortunately effective treatments to enhance healing and reduce scarring are still lacking. A deeper understanding of the physiology of normal repair and of the pathology of delayed healing is a prerequisite for the development of more effective therapeutic interventions. Transition from the inflammatory to the proliferative phase is a key step during healing and accumulating evidence associates a compromised transition with wound healing disorders. Thus, targeting factors that impact this phase transition may offer a rationale for therapeutic development. This review summarizes mechanisms regulating the inflammation–proliferation transition at cellular and molecular levels. We propose that identification of such mechanisms will reveal promising targets for development of more effective therapies.
Related collections
Most cited references204
- Record: found
- Abstract: found
- Article: not found
Wound healing--aiming for perfect skin regeneration.
- Record: found
- Abstract: found
- Article: not found
How matrix metalloproteinases regulate cell behavior.
- Record: found
- Abstract: found
- Article: not found