- Record: found
- Abstract: found
- Article: found
BRE modulates granulosa cell death to affect ovarian follicle development and atresia in the mouse
Read this article at
Abstract
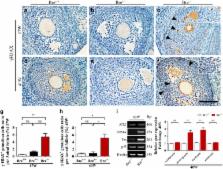
The BRE (brain and reproductive expression) gene, highly expressed in nervous and reproductive system organs, plays an important role in modulating DNA damage repair under stress response and pathological conditions. Folliculogenesis, a process that ovarian follicle develops into maturation, is closely associated with the interaction between somatic granulosa cell and oocyte. However, the regulatory role of BRE in follicular development remains undetermined. In this context, we found that BRE is normally expressed in the oocytes and granulosa cells from the primordial follicle stage. There was a reduction in follicles number of BRE mutant (BRE −/−) mice. It was attributed to increase the follicular atresia in ovaries, as a result of retarded follicular development. We established that cell proliferation was inhibited, while apoptosis was markedly increased in the granulosa cells in the absence of BRE. In addition, expressions of γ-H2AX (marker for showing DNA double-strand breaks) and DNA damage-relevant genes are both upregulated in BRE −/− mice. In sum, these results suggest that the absence of BRE, deficiency in DNA damage repair, causes increased apoptosis in granulosa cells, which in turn induces follicular atresia in BRE −/− mice.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Cell cycle regulation and neural differentiation.
- Record: found
- Abstract: found
- Article: not found
Premature ovarian failure in androgen receptor-deficient mice.
- Record: found
- Abstract: found
- Article: not found