- Record: found
- Abstract: found
- Article: found
miR-143 promotes angiogenesis and osteoblast differentiation by targeting HDAC7
Read this article at
Abstract
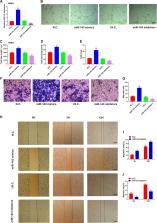
The regulation of bone formation and detailed mechanisms are still largely elusive, and the roles of microRNAs in this process have attracted much attention. Recently, a specific subtype of CD31 hiendomucin hi (CD31 hiEMCN hi) endothelium has been identified to promote bone formation, together with osteoblast development. However, the role of microRNA143 in the generation of CD31 hi EMCN hi endothelium and bone formation remains unknown. In this study, we found that miR-143 was expressed both in osteoblast cells and CD31 hiEMCN hi endothelial cells. Serum miR-143 level was negatively correlated with age in humans. Overexpression of miR-143 promoted osteoblast formation and angiogenic effects. Furthermore, CD31 hiEmcn hi vessels and osteoblast formation were significantly inhibited in miR-143 knockout mice. Mechanistically, inhibitor HDAC7 was directly targeted by miR-143 and knockdown of HDAC7 was found to rescue the function of miR-143 deficiency. Thus, miR-143 promotes angiogenesis coupling with osteoblast differentiation by targeting HDAC7, which may serve as a potential target in angiogenic and osteogenic diseases.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone.
- Record: found
- Abstract: found
- Article: not found
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
- Record: found
- Abstract: found
- Article: not found