- Record: found
- Abstract: found
- Article: found
Implementation of a Physiologically Based Pharmacokinetic Modeling Approach to Guide Optimal Dosing Regimens for Imatinib and Potential Drug Interactions in Paediatrics

Read this article at
Abstract
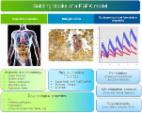
Long-term use of imatinib is effective and well-tolerated in children with chronic myeloid leukaemia (CML) yet defining an optimal dosing regimen for imatinib in younger patients is a challenge. The potential interactions between imatinib and coadministered drugs in this “special” population also remains largely unexplored. This study implements a physiologically based pharmacokinetic (PBPK) modeling approach to investigate optimal dosing regimens and potential drug interactions with imatinib in the paediatric population. A PBPK model for imatinib was developed in the Simcyp Simulator (version 17) utilizing in silico, in vitro drug metabolism, and in vivo pharmacokinetic data and verified using an independent set of published clinical pharmacokinetic data. The model was then extrapolated to children and adolescents (aged 2–18 years) by incorporating developmental changes in organ size and maturation of drug-metabolising enzymes and plasma protein responsible for imatinib disposition. The PBPK model described imatinib pharmacokinetics in adult and paediatric populations and predicted drug interaction with carbamazepine, a cytochrome P450 (CYP)3A4 and 2C8 inducer, with a good accuracy (evaluated by visual inspections of the simulation results and predicted pharmacokinetic parameters that were within 1.25-fold of the clinically observed values). The PBPK simulation suggests that the optimal dosing regimen range for imatinib is 230–340 mg/m 2/d in paediatrics, which is supported by the recommended initial dose for treatment of childhood CML. The simulations also highlighted that children and adults being treated with imatinib have similar vulnerability to CYP modulations. A PBPK model for imatinib was successfully developed with an excellent performance in predicting imatinib pharmacokinetics across age groups. This PBPK model is beneficial to guide optimal dosing regimens for imatinib and predict drug interactions with CYP modulators in the paediatric population.
Related collections
Most cited references106
- Record: found
- Abstract: found
- Article: not found
Impact of OATP transporters on pharmacokinetics.
- Record: found
- Abstract: found
- Article: not found
Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study.
- Record: found
- Abstract: found
- Article: found