- Record: found
- Abstract: found
- Article: found
Re-education of Tumor-Associated Macrophages by CXCR2 Blockade Drives Senescence and Tumor Inhibition in Advanced Prostate Cancer
Read this article at
Summary
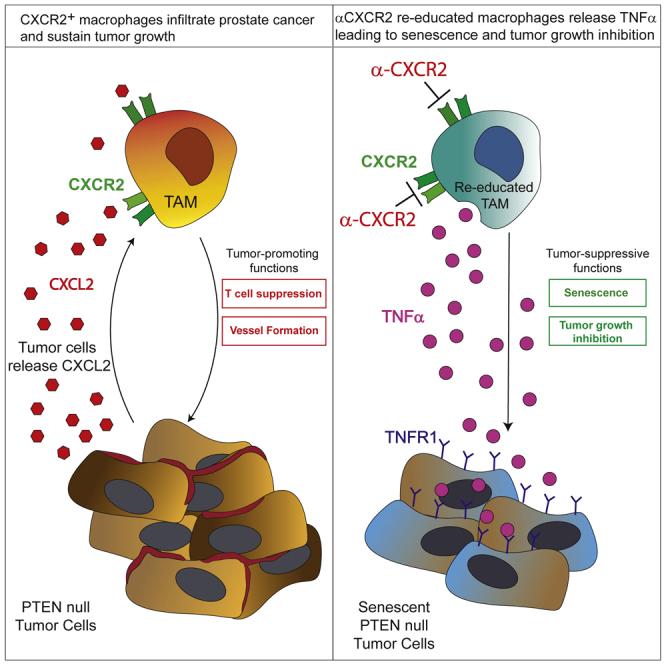
Tumor-associated macrophages (TAMs) represent a major component of the tumor microenvironment supporting tumorigenesis. TAMs re-education has been proposed as a strategy to promote tumor inhibition. However, whether this approach may work in prostate cancer is unknown. Here we find that Pten-null prostate tumors are strongly infiltrated by TAMs expressing C-X-C chemokine receptor type 2 (CXCR2), and activation of this receptor through CXCL2 polarizes macrophages toward an anti-inflammatory phenotype. Notably, pharmacological blockade of CXCR2 receptor by a selective antagonist promoted the re-education of TAMs toward a pro-inflammatory phenotype. Strikingly, CXCR2 knockout monocytes infused in Pten pc−/−; Trp53 pc−/− mice differentiated in tumor necrosis factor alpha (TNF-α)-releasing pro-inflammatory macrophages, leading to senescence and tumor inhibition. Mechanistically, PTEN-deficient tumor cells are vulnerable to TNF-α-induced senescence, because of an increase of TNFR1. Our results identify TAMs as targets in prostate cancer and describe a therapeutic strategy based on CXCR2 blockade to harness anti-tumorigenic potential of macrophages against this disease.
Graphical Abstract
Highlights
Abstract
Di Mitri et al. show that CXCR2 blockade in prostate cancer triggers TAMs re-education, leading to tumor inhibition. CXCR2-KO monocytes infused in Pten pc−/−; Trp53 pc−/− tumor-bearing mice differentiate into TNFα-releasing pro-inflammatory macrophages that induce senescence in tumor cells. PTEN-null tumors display higher sensitivity to TNF-α-induced senescence because of TNFR1 upregulation.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4.
- Record: found
- Abstract: found
- Article: not found