- Record: found
- Abstract: found
- Article: found
The lipid droplet—a well-connected organelle
Read this article at
Abstract
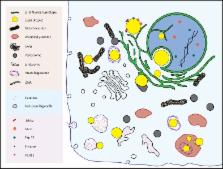
Our knowledge of inter-organellar communication has grown exponentially in recent years. This review focuses on the interactions that cytoplasmic lipid droplets have with other organelles. Twenty-five years ago droplets were considered simply particles of coalesced fat. Ten years ago there were hints from proteomics studies that droplets might interact with other structures to share lipids and proteins. Now it is clear that the droplets interact with many if not most cellular structures to maintain cellular homeostasis and to buffer against insults such as starvation. The evidence for this statement, as well as probes to understand the nature and results of droplet interactions, are presented.
Related collections
Most cited references103
- Record: found
- Abstract: found
- Article: not found
Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets.
- Record: found
- Abstract: found
- Article: not found