- Record: found
- Abstract: found
- Article: found
Communication Between Cardiomyocytes and Fibroblasts During Cardiac Ischemia/Reperfusion and Remodeling: Roles of TGF-β, CTGF, the Renin Angiotensin Axis, and Non-coding RNA Molecules
Read this article at
Abstract
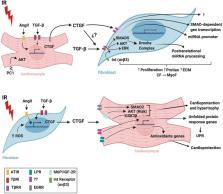
Communication between cells is a foundational concept for understanding the physiology and pathology of biological systems. Paracrine/autocrine signaling, direct cell-to-cell interplay, and extracellular matrix interactions are three types of cell communication that regulate responses to different stimuli. In the heart, cardiomyocytes, fibroblasts, and endothelial cells interact to form the cardiac tissue. Under pathological conditions, such as myocardial infarction, humoral factors released by these cells may induce tissue damage or protection, depending on the type and concentration of molecules secreted. Cardiac remodeling is also mediated by the factors secreted by cardiomyocytes and fibroblasts that are involved in the extensive reciprocal interactions between these cells. Identifying the molecules and cellular signal pathways implicated in these processes will be crucial for creating effective tissue-preserving treatments during or after reperfusion. Numerous therapies to protect cardiac tissue from reperfusion-induced injury have been explored, and ample pre-clinical research has attempted to identify drugs or techniques to mitigate cardiac damage. However, despite great success in animal models, it has not been possible to completely translate these cardioprotective effects to human applications. This review provides a current summary of the principal molecules, pathways, and mechanisms underlying cardiomyocyte and cardiac fibroblast crosstalk during ischemia/reperfusion injury. We also discuss pre-clinical molecules proposed as treatments for myocardial infarction and provide a clinical perspective on these potential therapeutic agents.
Related collections
Most cited references168
- Record: found
- Abstract: found
- Article: not found
Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis.
- Record: found
- Abstract: found
- Article: not found
The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis.
- Record: found
- Abstract: found
- Article: not found