- Record: found
- Abstract: found
- Article: found
Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps
Read this article at
Abstract
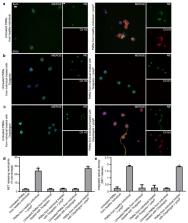
Neutrophils through the release of neutrophil extracellular traps (NETs) containing active tissue factor (TF) are key components of thrombo-inflammation. Platelets-neutrophils interplay in ST elevation myocardial infarction (STEMI) promotes NET formation via inorganic polyphosphates (polyP) released by thrombin-activated platelets. NETs, however, are also induced by biomaterials in a platelet-independent manner. Considering the possible pleiotropic effects of Ticagrelor beyond platelet inhibition and the clinical need for novel antithrombotic strategies targeting inflammation, we investigated the effects of Ticagrelor on polyP and stent-induced NETs in STEMI. Neutrophils from healthy individuals and patients receiving Ticagrelor were stimulated with polyP or drug-eluting stents (DES) to produce NETs. To induce TF expression, neutrophils were further incubated with plasma obtained from the infarct-related artery (IRA) of STEMI patients. The effects of Ticagrelor on NETs and TF loading were assessed using fluorescence microscopy, flow cytometry, myeloperoxidase(MPO)/DNA complex ELISA, and a Western blot. Ticagrelor interrupts platelet–neutrophil interaction by attenuating NETs induced by polyP. However, Ticagrelor does not affect polyP secretion from thrombin-activated platelets. Similarly, the intracellular production of TF in neutrophils triggered by IRA plasma is not hindered by Ticagrelor. Furthermore, DES induce NETs and synchronous stimulation with IRA plasma leads to the formation of thrombogenic TF-bearing NETs. Ticagrelor inhibits stent-induced NET release. These findings suggest a novel immune-modulatory effect of Ticagrelor when it attenuates the formation of thrombogenic NETs.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood.
- Record: found
- Abstract: found
- Article: not found
Netting neutrophils in autoimmune small-vessel vasculitis.

- Record: found
- Abstract: found
- Article: found