- Record: found
- Abstract: found
- Article: found
Distinct Dorsal and Ventral Hippocampal CA3 Outputs Govern Contextual Fear Discrimination

Read this article at
SUMMARY
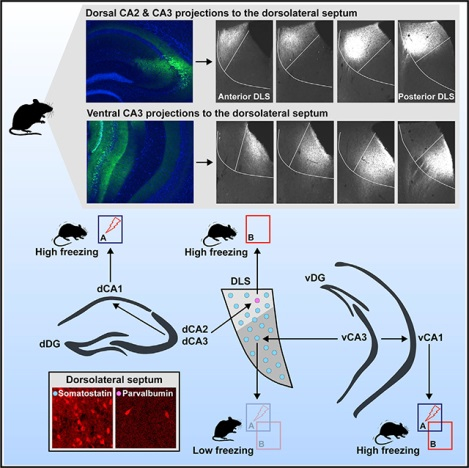
Considerable work emphasizes a role for hippocampal circuits in governing contextual fear discrimination. However· the intra- and extrahippocampal pathways that route contextual information to cortical and subcortical circuits to guide adaptive behavioral responses are poorly understood. Using terminal-specific optogenetic silencing in a contextual fear discrimination learning paradigm· we identify opposing roles for dorsal CA3-CA1 (dCA3-dCA1) projections and dorsal CA3-dorsolateral septum (dCA3-DLS) projections in calibrating fear responses to certain and ambiguous contextual threats· respectively. Ventral CA3-DLS (vCA3-DLS) projections suppress fear responses in both certain and ambiguous contexts· whereas ventral CA3-CA1 (vCA3-vCA1) projections promote fear responses in both these contexts. Lastly· using retrograde monosynaptic tracing· ex vivo electrophysiological recordings· and optogenetics,· we identify a sparse population of DLS parvalbumin (PV) neurons as putative relays of dCA3-DLS projections to diverse subcortical circuits. Taken together· these studies illuminate how distinct dCA3 and vCA3 outputs calibrate contextual fear discrimination.
Graphical Abstract
In Brief
Besnard et al. show that dorsal and ventral hippocampal CA3 projections to CA1 and dorsolateral septum (DLS) play distinct roles in calibration of contextual fear discrimination. DLS parvalbumin inhibitory neurons receive monosynaptic dorsal CA3 inputs and modulate fear responses in a context-specific manner.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex.
- Record: found
- Abstract: found
- Article: not found
Pattern separation in the dentate gyrus and CA3 of the hippocampus.
- Record: found
- Abstract: found
- Article: not found