- Record: found
- Abstract: found
- Article: found
Advances in Developing CAR T-Cell Therapy for HIV Cure
Read this article at
Abstract
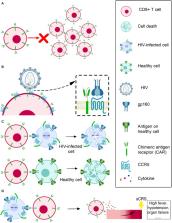
Acquired immune deficiency syndrome (AIDS), which is caused by HIV infection, is an epidemic disease that has killed millions of people in the last several decades. Although combination antiretroviral therapy (cART) has enabled tremendous progress in suppressing HIV replication, it fails to eliminate HIV latently infected cells, and infected individuals remain HIV positive for life. Lifelong antiretroviral therapy is required to maintain control of virus replication, which may result in significant problems, including long-term toxicity, high cost, and stigma. Therefore, novel therapeutic strategies are urgently needed to eliminate the viral reservoir in the host for HIV cure. In this review, we compare several potential strategies regarding HIV cure and focus on how we might utilize chimeric antigen receptor-modified T cells (CAR T) as a therapy to cure HIV infection.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy.
- Record: found
- Abstract: found
- Article: not found
CD22-CAR T Cells Induce Remissions in CD19-CAR Naïve and Resistant B-ALL
- Record: found
- Abstract: found
- Article: not found