- Record: found
- Abstract: found
- Article: found
Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway
Read this article at
Abstract
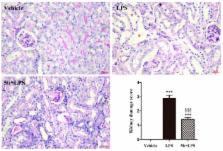
Treatment of septic acute kidney injury (AKI) has still been beyond satisfaction, although anti-inflammatory therapy is beneficial for sepsis-induced AKI. Compound 5b was derived from natural pyranochalcones and exhibited potent anti-inflammatory activity in adjuvant-induced arthritis. In this study, we aimed to investigate the renoprotective effects and potential mechanism of 5b against lipopolysaccharide (LPS)-induced AKI. C57BL/6 mice and human renal proximal tubule cell line (HK-2 cell) were treated with LPS, respectively. Compound 5b was orally administrated at a dose of 25 mg/kg/day for 5 days before LPS (10 mg/kg) intraperitoneal injection. Cells were pretreated with 25 μg/mL 5b for 30 min before LPS (1 μg/mL) treatment. Pretreatment with 5b markedly alleviated tubular injury and renal dysfunction in LPS-induced AKI. The expression of IL-1β, IL-6, and TNF-α both in renal tissue of AKI mice and in the LPS-stimulated HK-2 cell culture medium were reduced by 5b treatment ( p < 0.05). The results of immunohistochemistry staining showed that 5b reduced the expression of NF-κB p65 in kidneys. Similarly, 5b decreased the LPS-induced levels of NF-κB p65 and TLR4 proteins in kidneys and HK-2 cells. These data demonstrated that a potent pyranochalcone derivative, 5b, exhibited renoprotective effect against LPS-induced AKI, which was associated with anti-inflammatory activity by inhibiting the TLR4/NF-κB pathway.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Acute Kidney Injury.
- Record: found
- Abstract: found
- Article: not found