- Record: found
- Abstract: found
- Article: not found
Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium
Read this article at
Abstract
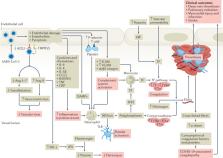
Coronavirus disease 2019 (COVID-19) predisposes patients to thrombotic and thromboembolic events, owing to excessive inflammation, endothelial cell activation and injury, platelet activation and hypercoagulability. Patients with COVID-19 have a prothrombotic or thrombophilic state, with elevations in the levels of several biomarkers of thrombosis, which are associated with disease severity and prognosis. Although some biomarkers of COVID-19-associated coagulopathy, including high levels of fibrinogen and d-dimer, were recognized early during the pandemic, many new biomarkers of thrombotic risk in COVID-19 have emerged. In this Consensus Statement, we delineate the thrombotic signature of COVID-19 and present the latest biomarkers and platforms to assess the risk of thrombosis in these patients, including markers of platelet activation, platelet aggregation, endothelial cell activation or injury, coagulation and fibrinolysis as well as biomarkers of the newly recognized post-vaccine thrombosis with thrombocytopenia syndrome. We then make consensus recommendations for the clinical use of these biomarkers to inform prognosis, assess disease acuity, and predict thrombotic risk and in-hospital mortality. A thorough understanding of these biomarkers might aid risk stratification and prognostication, guide interventions and provide a platform for future research.
Abstract
In this Consensus Statement, the authors delineate the thrombotic signature of COVID-19 and present the latest biomarkers and platforms to assess thrombotic risk in these patients. Consensus recommendations are made about the clinical use of these biomarkers to inform prognosis, assess disease acuity, and predict thrombosis and in-hospital mortality.
Related collections
Most cited references211
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: not found
