- Record: found
- Abstract: found
- Article: found
The Complexities of Organ Crosstalk in Phosphate Homeostasis: Time to Put Phosphate Sensing Back in the Limelight
Read this article at
Abstract
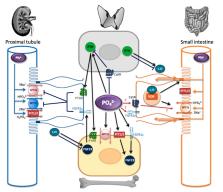
Phosphate homeostasis is essential for health and is achieved via interaction between the bone, kidney, small intestine, and parathyroid glands and via intricate processes involving phosphate transporters, phosphate sensors, and circulating hormones. Numerous genetic and acquired disorders are associated with disruption in these processes and can lead to significant morbidity and mortality. The role of the kidney in phosphate homeostasis is well known, although it is recognized that the cellular mechanisms in murine models and humans are different. Intestinal phosphate transport also appears to differ in humans and rodents, with recent studies demonstrating a dominant role for the paracellular pathway. The existence of phosphate sensing has been acknowledged for decades; however, the underlying molecular mechanisms are poorly understood. At least three phosphate sensors have emerged. PiT2 and FGFR1c both act as phosphate sensors controlling Fibroblast Growth Factor 23 secretion in bone, whereas the calcium-sensing receptor controls parathyroid hormone secretion in response to extracellular phosphate. All three of the proposed sensors are expressed in the kidney and intestine but their exact function in these organs is unknown. Understanding organ interactions and the mechanisms involved in phosphate sensing requires significant research to develop novel approaches for the treatment of phosphate homeostasis disorders.
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
The Fibroblast Growth Factor signaling pathway
- Record: found
- Abstract: found
- Article: not found
KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD).
- Record: found
- Abstract: found
- Article: not found