- Record: found
- Abstract: found
- Article: found
Blockade of ARHGAP11A reverses malignant progress via inactivating Rac1B in hepatocellular carcinoma
Read this article at
Abstract
Background
The molecular signaling events involving in high malignancy and poor prognosis of hepatocellular carcinoma (HCC) are extremely complicated. Blockade of currently known targets has not yet led to successful clinical outcome. More understanding about the regulatory mechanisms in HCC is necessary for developing new effective therapeutic strategies for HCC patients.
Methods
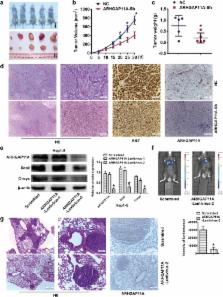
The expression of Rho GTPase-activating protein 11A (ARHGAP11A) was examined in human normal liver and HCC tissues. The correlations between ARHGAP11A expression and clinicopathological stage or prognosis in HCC patients were analyzed. ARHGAP11A was downregulated to determine its role in the proliferation, invasion, migration, epithelial-to-mesenchymal transition (EMT) development, and regulatory signaling of HCC cells in vitro and in vivo.
Results
ARHGAP11A exhibited high expression in HCC, and was significantly correlated with clinicopathological stage and prognosis in HCC patients. Moreover, ARHGAP11A facilitated Hep3B and MHCC97-H cell proliferation, invasion, migration and EMT development in vitro. ARHGAP11A knockdown significantly inhibited the in vivo growth and metastasis of HCC cells. Furthermore, ARHGAP11A directly interacted with Rac1B independent of Rho GTPase- activating activity. Rac1B blockade effectively interrupted ARHGAP11A-elicited HCC malignant phenotype. Meanwhile, upregulation of Rac1B reversed ARHGAP11A knockdown mediated mesenchymal-to-epithelial transition (MET) development in HCC cells.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
New signals from the invasive front.
- Record: found
- Abstract: found
- Article: not found
Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation.
- Record: found
- Abstract: found
- Article: not found