- Record: found
- Abstract: found
- Article: found
The positive efficacy of dexmedetomidine on the clinical outcomes of patients undergoing renal transplantation: evidence from meta-analysis
Read this article at
Abstract
Introduction: Whether dexmedetomidine (DEX), an anesthetic adjuvant, can improve renal transplant outcomes is not clear.
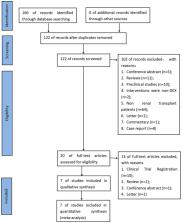
Methods: We systematically identified clinical trials in which DEX was administered in renal transplantation (RT). On November 1, 2022, we searched The Cochrane Library, MEDLINE, EMBASE and https://www.clinicaltrials.gov/. The main outcomes were delayed graft function and acute rejection.
Results: A total of seven studies were included in the meta-analysis. The results showed that compared with the control, DEX significantly reduced the occurrence of delayed graft function (RR 0.76; 95% CI 0.60–0.98), short-term serum creatinine [postoperative day (POD) 2: (MD −22.82; 95% CI −42.01 – −3.64)] and blood urea nitrogen [POD 2: (MD −2.90; 95% CI −5.10 – −0.70); POD 3: (MD 2.07; 95% CI −4.12 – −0.02)] levels, postoperative morphine consumption (MD −4.27; 95% CI −5.92 – −2.61) and the length of hospital stay (MD −0.85; 95% CI−1.47 – −0.23). However, DEX did not reduce the risk of postoperative acute rejection (RR 0.75; 95% CI 0.45–1.23). The results of the subgroup analysis showed that country type, donor type, and average age had a certain impact on the role of DEX.
Conclusions: DEX may improve the short-term clinical outcome of RT and shorten the length of hospital stay of patients.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.

- Record: found
- Abstract: found
- Article: found
Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range
- Record: found
- Abstract: not found
- Article: not found