- Record: found
- Abstract: found
- Article: found
Roles of HIF and 2-Oxoglutarate-Dependent Dioxygenases in Controlling Gene Expression in Hypoxia
Read this article at
Abstract
Simple Summary
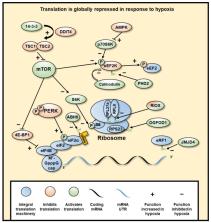
Hypoxia—reduction in oxygen availability—plays key roles in both physiological and pathological processes. Given the importance of oxygen for cell and organism viability, mechanisms to sense and respond to hypoxia are in place. A variety of enzymes utilise molecular oxygen, but of particular importance to oxygen sensing are the 2-oxoglutarate (2-OG) dependent dioxygenases (2-OGDs). Of these, Prolyl-hydroxylases have long been recognised to control the levels and function of Hypoxia Inducible Factor (HIF), a master transcriptional regulator in hypoxia, via their hydroxylase activity. However, recent studies are revealing that such dioxygenases are involved in almost all aspects of gene regulation, including chromatin organisation, transcription and translation.
Abstract
Hypoxia—reduction in oxygen availability—plays key roles in both physiological and pathological processes. Given the importance of oxygen for cell and organism viability, mechanisms to sense and respond to hypoxia are in place. A variety of enzymes utilise molecular oxygen, but of particular importance to oxygen sensing are the 2-oxoglutarate (2-OG) dependent dioxygenases (2-OGDs). Of these, Prolyl-hydroxylases have long been recognised to control the levels and function of Hypoxia Inducible Factor (HIF), a master transcriptional regulator in hypoxia, via their hydroxylase activity. However, recent studies are revealing that dioxygenases are involved in almost all aspects of gene regulation, including chromatin organisation, transcription and translation. We highlight the relevance of HIF and 2-OGDs in the control of gene expression in response to hypoxia and their relevance to human biology and health.
Related collections
Most cited references319
- Record: found
- Abstract: found
- Article: not found
HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing.
- Record: found
- Abstract: found
- Article: not found
A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation
- Record: found
- Abstract: found
- Article: not found