- Record: found
- Abstract: found
- Article: not found
Ankyrin-B Is Required for Intracellular Sorting of Structurally Diverse Ca 2+ Homeostasis Proteins
Read this article at
Abstract
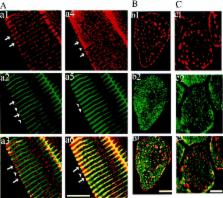
This report describes a congenital myopathy and major loss of thymic lymphocytes in ankyrin-B (−/−) mice as well as dramatic alterations in intracellular localization of key components of the Ca 2+ homeostasis machinery in ankyrin-B (−/−) striated muscle and thymus. The sacoplasmic reticulum (SR) and SR/T-tubule junctions are apparently preserved in a normal distribution in ankyrin-B (−/−) skeletal muscle based on electron microscopy and the presence of a normal pattern of triadin and dihydropyridine receptor. Therefore, the abnormal localization of SR/ER Ca ATPase (SERCA) and ryanodine receptors represents a defect in intracellular sorting of these proteins in skeletal muscle. Extrapolation of these observations suggests defective targeting as the basis for abnormal localization of ryanodine receptors, IP3 receptors and SERCA in heart, and of IP3 receptors in the thymus of ankyrin-B (−/−) mice. Mis-sorting of SERCA 2 and ryanodine receptor 2 in ankyrin-B (−/−) cardiomyocytes is rescued by expression of 220-kD ankyrin-B, demonstrating that lack of the 220-kD ankyrin-B polypeptide is the primary defect in these cells. Ankyrin-B is associated with intracellular vesicles, but is not colocalized with the bulk of SERCA 1 or ryanodine receptor type 1 in skeletal muscle. These data provide the first evidence of a physiological requirement for ankyrin-B in intracellular targeting of the calcium homeostasis machinery of striated muscle and immune system, and moreover, support a catalytic role that does not involve permanent stoichiometric complexes between ankyrin-B and targeted proteins. Ankyrin-B is a member of a family of adapter proteins implicated in restriction of diverse proteins to specialized plasma membrane domains. Similar mechanisms involving ankyrins may be essential for segregation of functionally defined proteins within specialized regions of the plasma membrane and within the Ca 2+ homeostasis compartment of the ER.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
AnkyrinG Is Required for Clustering of Voltage-gated Na Channels at Axon Initial Segments and for Normal Action Potential Firing
- Record: found
- Abstract: found
- Article: not found
Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions.
- Record: found
- Abstract: found
- Article: not found