- Record: found
- Abstract: found
- Article: found
Physical exercise promotes astrocyte coverage of microvessels in a model of chronic cerebral hypoperfusion
Read this article at
Abstract
Background
Brain circulation disorders such as chronic cerebral hypoperfusion have been associated with a decline in cognitive function during the development of dementia. Astrocytes together with microglia participate in the immune response in the CNS and make them potential sentinels in the brain parenchyma. In addition, astrocytes coverage integrity has been related to brain homeostasis. Currently, physical exercise has been proposed as an effective intervention to promote brain function improvement. However, the neuroprotective effects of early physical exercise on the astrocyte communication with the microcirculation and the microglial activation in a chronic cerebral hypoperfusion model are still unclear. The aim of this study was to investigate the impact of early intervention with physical exercise on cognition, brain microcirculatory, and inflammatory parameters in an experimental model of chronic cerebral hypoperfusion induced by permanent bilateral occlusion of the common carotid arteries (2VO).
Methods
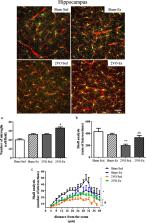
Wistar rats aged 12 weeks were randomly divided into four groups: Sham-sedentary group (Sham-Sed), Sham-exercised group (Sham-Ex), 2VO-sedentary group (2VO-Sed), and 2VO-exercised group (2VO-Ex). The early intervention with physical exercise started 3 days after 2VO or Sham surgery during 12 weeks. Then, the brain functional capillary density and endothelial-leukocyte interactions were evaluated by intravital microscopy; cognitive function was evaluated by open-field test; hippocampus postsynaptic density protein 95 and synaptophysin were evaluated by western blotting; astrocytic coverage of the capillaries, microglial activation, and structural capillary density were evaluated by immunohistochemistry.
Results
Early moderate physical exercise was able to normalize functional capillary density and reduce leukocyte rolling in the brain of animals with chronic cerebral hypoperfusion. These effects were accompanied by restore synaptic protein and the improvement of cognitive function. In addition, early moderate exercise improves astrocytes coverage in blood vessels of the cerebral cortex and hippocampus, decreases microglial activation in the hippocampus, and improves structural capillaries in the hippocampus.
Conclusions
Microcirculatory and inflammatory changes in the brain appear to be involved in triggering a cognitive decline in animals with chronic cerebral ischemia. Therefore, early intervention with physical exercise may represent a preventive approach to neurodegeneration caused by chronic cerebral hypoperfusion.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Cardiovascular fitness, cortical plasticity, and aging.
- Record: found
- Abstract: found
- Article: not found
Interplay of hippocampus and prefrontal cortex in memory.

- Record: found
- Abstract: found
- Article: found