- Record: found
- Abstract: found
- Article: found
Neuroanatomical and Microglial Alterations in the Striatum of Levodopa-Treated, Dyskinetic Hemi-Parkinsonian Rats
Read this article at
Abstract
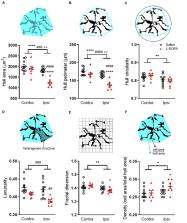
Dyskinesia associated with chronic levodopa treatment in Parkinson’s disease is associated with maladaptive striatal plasticity. The objective of this study was to examine whether macroscale structural changes, as captured by magnetic resonance imaging (MRI) accompany this plasticity and to identify plausible cellular contributors in a rodent model of levodopa-induced dyskinesia. Adult male Sprague-Dawley rats were rendered hemi-parkinsonian by stereotaxic injection of 6-hydroxydopamine into the left medial forebrain bundle prior to chronic treatment with saline (control) or levodopa to induce abnormal involuntary movements (AIMs), reflective of dyskinesia. Perfusion-fixed brains underwent ex vivo structural MRI before sectioning and staining for cellular markers. Chronic treatment with levodopa induced significant AIMs ( p < 0.0001 versus saline). The absolute volume of the ipsilateral, lesioned striatum was increased in levodopa-treated rats resulting in a significant difference in percentage volume change when compared to saline-treated rats ( p < 0.01). Moreover, a significant positive correlation was found between this volume change and AIMs scores for individual levodopa-treated rats ( r = 0.96; p < 0.01). The density of Iba1+ cells was increased within the lesioned versus intact striatum ( p < 0.01) with no difference between treatment groups. Conversely, Iba1+ microglia soma size was significantly increased ( p < 0.01) in the lesioned striatum of levodopa-treated but not saline-treated rats. Soma size was not, however, significantly correlated with either AIMs or MRI volume change. Although GFAP+ astrocytes were elevated in the lesioned versus intact striatum ( p < 0.001), there was no difference between treatment groups. No statistically significant effects of either lesion or treatment on RECA1, a marker for blood vessels, were observed. Collectively, these data suggest chronic levodopa treatment in 6-hydroxydopamine lesioned rats is associated with increased striatal volume that correlates with the development of AIMs. The accompanying increase in number and size of microglia, however, cannot alone explain this volume expansion. Further multi-modal studies are warranted to establish the brain-wide effects of chronic levodopa treatment.
Related collections
Most cited references60

- Record: found
- Abstract: found
- Article: found
Quantitating the subtleties of microglial morphology with fractal analysis
- Record: found
- Abstract: found
- Article: not found
Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia
- Record: found
- Abstract: found
- Article: not found