- Record: found
- Abstract: found
- Article: not found
Bromelain Surface Modification Increases the Diffusion of Silica Nanoparticles in the Tumor Extracellular Matrix
Read this article at
Abstract

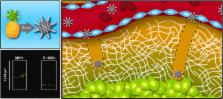
Tumor extracellular matrix (ECM) represents a major obstacle to the diffusion of therapeutics and drug delivery systems in cancer parenchyma. This biological barrier limits the efficacy of promising therapeutic approaches including the delivery of siRNA or agents intended for thermoablation. After extravasation due to the enhanced penetration and retention effect of tumor vasculature, typical nanotherapeutics are unable to reach the nonvascularized and anoxic regions deep within cancer parenchyma. Here, we developed a simple method to provide mesoporous silica nanoparticles (MSN) with a proteolytic surface. To this extent, we chose to conjugate MSN to Bromelain (Br–MSN), a crude enzymatic complex, purified from pineapple stems, that belongs to the peptidase papain family. This surface modification increased particle uptake in endothelial, macrophage, and cancer cell lines with minimal impact on cellular viability. Most importantly Br–MSN showed an increased ability to digest and diffuse in tumor ECM in vitro and in vivo.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance.
- Record: found
- Abstract: found
- Article: not found
Nanoparticle delivery of cancer drugs.
- Record: found
- Abstract: found
- Article: not found
