- Record: found
- Abstract: found
- Article: not found
Egyptian perspectives on potential risk of paracetamol/acetaminophen-induced toxicities: Lessons learnt during COVID-19 pandemic

Read this article at
Abstract
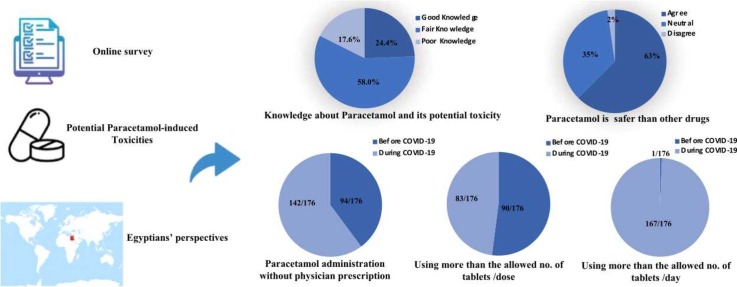
Paracetamol/Acetaminophen was widely used as a first-line antipyretic and analgesic for COVID-19 patients without giving any attention to the potential risk of related toxicities. A survey was conducted on 176 Egyptians using an online survey portal to assess their knowledge, and attitude regarding potential risk of paracetamol toxicities and whether COVID-19 pandemic affected their practices regarding safe use of paracetamol. The self-administered questionnaire was developed by the researchers and was validated by expert opinions. A pilot testing of the questionnaire was done. Alpha Cronbach test used to assess the internal consistency reliability of the survey revealed good reliability. Overall percent-score revealed that only 24.4% of participants had good knowledge about paracetamol and its related potential toxicities. 62.5% of participants considered paracetamol safer than other medications of the same indications. 42.6% of participants could advise others to use paracetamol without prescription. According to the participants' responses, physicians were less concerned to give instructions about possibility of overdosage. Our results also revealed that participants’ administration of paracetamol without physician prescription was more during COVID-19. Practice of paracetamol administration more than the allowed number of tablets/day was significantly more evident during the pandemic. We concluded that the unsupervised use of paracetamol is an alarming sign that should be addressed as this could lead to a high rate of accidental paracetamol toxicity. A lesson learnt from COVID-19 pandemic is the need to implement behavior change measures to mitigate the risk of accidental paracetamol toxicity.
Graphical Abstract
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus
- Record: found
- Abstract: found
- Article: not found
Origin and evolution of pathogenic coronaviruses

- Record: found
- Abstract: found
- Article: found