- Record: found
- Abstract: found
- Article: found
Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing
Read this article at
Abstract
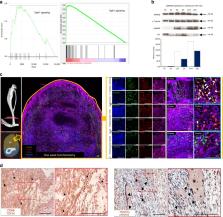
Heterotopic ossification (HO) is an aberrant regenerative process with ectopic bone induction in response to musculoskeletal trauma, in which mesenchymal stem cells (MSC) differentiate into osteochondrogenic cells instead of myocytes or tenocytes. Despite frequent cases of hospitalized musculoskeletal trauma, the inflammatory responses and cell population dynamics that regulate subsequent wound healing and tissue regeneration are still unclear. Here we examine, using a mouse model of trauma-induced HO, the local microenvironment of the initial post-injury inflammatory response. Single cell transcriptome analyses identify distinct monocyte/macrophage populations at the injury site, with their dynamic changes over time elucidated using trajectory analyses. Mechanistically, transforming growth factor beta-1 (TGFβ1)-producing monocytes/macrophages are associated with HO and aberrant chondrogenic progenitor cell differentiation, while CD47-activating peptides that reduce systemic macrophage TGFβ levels and help ameliorate HO. Our data thus implicate CD47 activation as a therapeutic approach for modulating monocyte/macrophage phenotypes, MSC differentiation and HO formation during wound healing.
Abstract
Aberrant tissue repair may result in heterotopic ossification (HO), but how this process is regulated by local inflammatory responses is still unclear. Here the authors show, using a mouse burn/trauma model, that TGFβ-producing monocytes/macrophages at the injury site contribute to HO induction, while CD47 activation helps antagonize this process.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction
- Record: found
- Abstract: found
- Article: not found
Colony-stimulating factor-1 in immunity and inflammation.
- Record: found
- Abstract: found
- Article: not found