- Record: found
- Abstract: found
- Article: found
PBPK Models for CYP3A4 and P‐gp DDI Prediction: A Modeling Network of Rifampicin, Itraconazole, Clarithromycin, Midazolam, Alfentanil, and Digoxin
Read this article at
Abstract
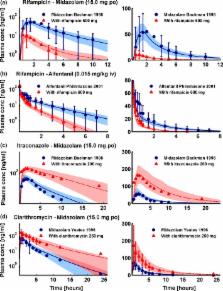
According to current US Food and Drug Administration ( FDA) and European Medicines Agency ( EMA) guidance documents, physiologically based pharmacokinetic ( PBPK) modeling is a powerful tool to explore and quantitatively predict drug‐drug interactions ( DDIs) and may offer an alternative to dedicated clinical trials. This study provides whole‐body PBPK models of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin within the Open Systems Pharmacology ( OSP) Suite. All models were built independently, coupled using reported interaction parameters, and mutually evaluated to verify their predictive performance by simulating published clinical DDI studies. In total, 112 studies were used for model development and 57 studies for DDI prediction. 93% of the predicted area under the plasma concentration‐time curve ( AUC) ratios and 94% of the peak plasma concentration (C max) ratios are within twofold of the observed values. This study lays a cornerstone for the qualification of the OSP platform with regard to reliable PBPK predictions of enzyme‐mediated and transporter‐mediated DDIs during model‐informed drug development. All presented models are provided open‐source and transparently documented.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin.
- Record: found
- Abstract: found
- Article: found
Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model
- Record: found
- Abstract: found
- Article: not found