- Record: found
- Abstract: found
- Article: not found
Worse outcome and distinct mutational pattern in follicular lymphoma with anti-HBc positivity
Read this article at
Key Points
Abstract
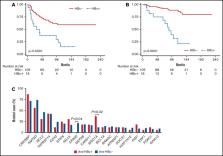
Epidemiological studies have demonstrated the association between hepatitis B virus (HBV) infection and B-cell non–Hodgkin lymphoma (NHL), mainly for diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). We studied a cohort of 121 patients with FL for HBV infection status, clinical features, and gene mutational profile. Anti-HBc was detectable in 16 patients (13.2%), although all had undetectable HBV DNA. Anti-HBcore + (anti-HBc +) cases presented with older age at diagnosis than anti-HBc − cases (68.1 vs 57.2 years; P = .007) and higher β2-microglobulin (56.3% vs 28.9%; P = .04). All patients included in the study fulfilled criteria for treatment and received therapy with rituximab or rituximab-containing chemotherapy. There were no episodes of HBV reactivation or HBV hepatitis during treatment and/or maintenance. Remarkably, anti-HBc + patients had significantly lower 10-year progression-free survival (PFS; 12.9% vs 58.3%; P < .0001) and overall survival (OS; 22.0% vs 86.2%; P < .0001), that remained at multivariate analysis. Gene mutational profiling of all cases showed that anti-HBc + cases had higher incidence of ARID1A mutations and absence of EP300 mutations, 2 key epigenetic regulators in FL. Overall, our study shows that FL patients with resolved HBV infection have a worse outcome independently of other well-known clinical risk factors and a distinct gene mutational profile.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma
- Record: found
- Abstract: found
- Article: not found
Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry.
- Record: found
- Abstract: found
- Article: not found
