- Record: found
- Abstract: found
- Article: found
Impact of levothyroxine therapy on obstetric, neonatal and childhood outcomes in women with subclinical hypothyroidism diagnosed in pregnancy: a systematic review and meta-analysis of randomised controlled trials
Read this article at
Abstract
Objective
To determine in women with subclinical hypothyroidism diagnosed in pregnancy whether levothyroxine treatment compared with control, impacts important obstetrical or childhood outcomes (specifically IQ) in randomised controlled trials.
Study eligibility criteria
Randomised trials which met all the following were included: (1) reported original data of women with subclinical hypothyroidism diagnosed in pregnancy (by any prespecified study definition); (2) randomised to either levothyroxine or control (placebo or no treatment); (3) reported obstetrical outcomes and/or childhood neurodevelopmental outcomes and (4) published from 1980 to January 2018 in either English or French language.
Data sources
Medline, EMBASE, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov.
Outcome measures
Obstetrical, neonatal and childhood outcomes including: miscarriage, gestational hypertension, pre-eclampsia, preterm delivery, mode of delivery, neonatal intensive care unit admission, birth weight, gestational age at delivery, childhood IQ and neurodevelopmental scores.
Risk of bias assessment
Cochrane Risk of Bias Tool (Modified) for Quality Assessment of Randomised Controlled Trials
Results
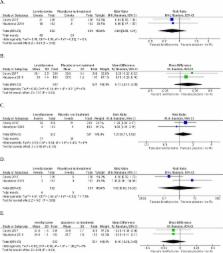
Three trials of low to unclear risk of bias with 1837 participants were included. Two studies were meta-analysed for maternal and neonatal outcomes and two studies for childhood IQ. No statistically significant differences were found for any clinical outcomes with levothyroxine therapy compared with control.
Conclusions
This review, based on three randomised trials in women with subclinical hypothyroidism diagnosed in pregnancy, found no evidence of benefit of levothyroxine therapy on obstetrical, neonatal, childhood IQ or neurodevelopmental outcomes. Current trial evidence does not support the treatment of subclinical hypothyroidism diagnosed in pregnancy.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline.
- Record: found
- Abstract: found
- Article: found
2014 European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and in Children
- Record: found
- Abstract: found
- Article: not found