- Record: found
- Abstract: found
- Article: found
Advances in Osteosarcoma
Read this article at
Abstract
Purpose of Review
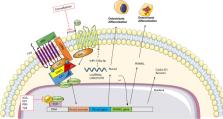
This article gives a brief overview of the most recent developments in osteosarcoma treatment, including targeting of signaling pathways, immune checkpoint inhibitors, drug delivery strategies as single or combined approaches, and the identification of new therapeutic targets to face this highly heterogeneous disease.
Recent Findings
Osteosarcoma is one of the most common primary malignant bone tumors in children and young adults, with a high risk of bone and lung metastases and a 5-year survival rate around 70% in the absence of metastases and 30% if metastases are detected at the time of diagnosis. Despite the novel advances in neoadjuvant chemotherapy, the effective treatment for osteosarcoma has not improved in the last 4 decades. The emergence of immunotherapy has transformed the paradigm of treatment, focusing therapeutic strategies on the potential of immune checkpoint inhibitors. However, the most recent clinical trials show a slight improvement over the conventional polychemotherapy scheme.
Related collections
Most cited references118
- Record: found
- Abstract: found
- Article: not found
The human tumor microbiome is composed of tumor type–specific intracellular bacteria
- Record: found
- Abstract: found
- Article: not found
Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial.

- Record: found
- Abstract: found
- Article: found