- Record: found
- Abstract: found
- Article: found
Non-Genomic Effects of Xenoestrogen Mixtures
Read this article at
Abstract
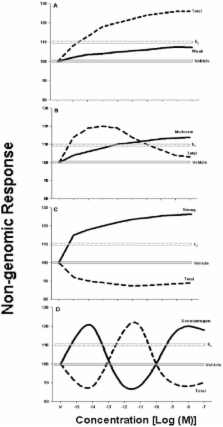
Xenoestrogens (XEs) are chemicals derived from a variety of natural and anthropogenic sources that can interfere with endogenous estrogens by either mimicking or blocking their responses via non-genomic and/or genomic signaling mechanisms. Disruption of estrogens’ actions through the less-studied non-genomic pathway can alter such functional end points as cell proliferation, peptide hormone release, catecholamine transport, and apoptosis, among others. Studies of potentially adverse effects due to mixtures and to low doses of endocrine-disrupting chemicals have recently become more feasible, though few so far have included actions via the non-genomic pathway. Physiologic estrogens and XEs evoke non-monotonic dose responses, with different compounds having different patterns of actions dependent on concentration and time, making mixture assessments all the more challenging. In order to understand the spectrum of toxicities and their mechanisms, future work should focus on carefully studying individual and mixture components across a range of concentrations and cellular pathways in a variety of tissue types.
Related collections
Most cited references142
- Record: found
- Abstract: found
- Article: not found
Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis.
- Record: found
- Abstract: found
- Article: not found
Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta.
- Record: found
- Abstract: not found
- Article: not found