- Record: found
- Abstract: found
- Article: found
Phosphorylated SATB1 is associated with the progression and prognosis of glioma

Read this article at
Abstract
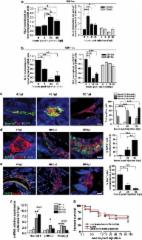
Special AT-rich sequence-binding protein 1 (SATB1) is a global chromatin organizer and gene regulator, and high expression of SATB1 is associated with progression and poor prognosis in several malignancies. Here, we examine the expression pattern of SATB1 in glioma. Microarray analysis of 127 clinical samples showed that SATB1 mRNA was expressed at lower levels in highly malignant glioblastoma multiforme (GBM) than in low-grade glioma and normal brain tissue. This result was further confirmed by real-time RT-PCR in the clinical samples, three GBM cell lines, primary SU3 glioma cells and tumor cells harvested by laser-capture microdissection. Consistent with the mRNA levels, SATB1 protein expression was downregulated in high-grade glioma, as shown by western blotting. However, phospho-SATB1 levels showed an opposite pattern, with a significant increase in these tumors. Immunohistochemical analysis of phospho-SATB1 expression in tissue microarrays with tumors from 122 glioma cases showed that phospho-SATB1 expression was significantly associated with high histological grade and poor survival by Kaplan–Meier analysis. In vitro transfection analysis showed that phospho-SATB1 DNA binding has a key role in regulating the proliferation and invasion of glioma cells. The effect of SATB1 in glioma cell is mainly histone deacetylase (HDAC1)-dependent. We conclude that phospho-SATB1, but not SATB1 mRNA expression, is associated with the progression and prognosis of glioma. By interaction with HDAC1, phospho-SATB1 contributes to the invasive and proliferative phenotype of GBM cells.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia.

- Record: found
- Abstract: found
- Article: found
Astrocytes Enhance the Invasion Potential of Glioblastoma Stem-Like Cells
- Record: found
- Abstract: found
- Article: found