- Record: found
- Abstract: found
- Article: found
Embryonic expression patterns and phylogenetic analysis of panarthropod sox genes: insight into nervous system development, segmentation and gonadogenesis
Read this article at
Abstract
Background
Sox ( Sry-related high-mobility-group b ox) genes represent important factors in animal development. Relatively little, however, is known about the embryonic expression patterns and thus possible function(s) of Sox genes during ontogenesis in panarthropods (Arthropoda+Tardigrada+Onychophora). To date, studies have been restricted exclusively to higher insects, including the model system Drosophila melanogaster, with no comprehensive data available for any other arthropod group, or any tardigrade or onychophoran.
Results
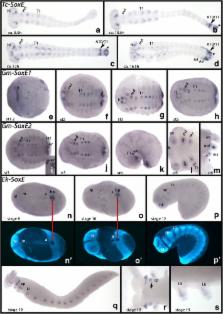
This study provides a phylogenetic analysis of panarthropod Sox genes and presents the first comprehensive analysis of embryonic expression patterns in the flour beetle Tribolium castaneum (Hexapoda), the pill millipede Glomeris marginata (Myriapoda), and the velvet worm, Euperipatoides kanangrensis (Onychophora). 24 Sox genes were identified and investigated: 7 in Euperipatoides, 8 in Glomeris, and 9 in Tribolium. Each species possesses at least one ortholog of each of the five expected Sox gene families, B, C, D, E, and F, many of which are differentially expressed during ontogenesis.
Conclusion
Sox gene expression (and potentially function) is highly conserved in arthropods and their closest relatives, the onychophorans. Sox B, C and D class genes appear to be crucial for nervous system development, while the Sox B genes Dichaete ( D) and Sox21b likely play an additional conserved role in panarthropod segmentation. The Sox B gene Sox21a likely has a conserved function in foregut and Malpighian tubule development, at least in Hexapoda. The data further suggest that Sox D and E genes are involved in mesoderm differentiation, and that Sox E genes are involved in gonadal development.
The new data expand our knowledge about the expression and implied function of Sox genes to Mandibulata (Myriapoda+Pancrustacea) and Panarthropoda (Arthropoda+Onychophora).
Related collections
Most cited references87
- Record: found
- Abstract: found
- Article: not found
Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators.
- Record: found
- Abstract: found
- Article: not found
Genomic data do not support comb jellies as the sister group to all other animals.
- Record: found
- Abstract: found
- Article: found