- Record: found
- Abstract: found
- Article: found
Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future
Read this article at
Abstract
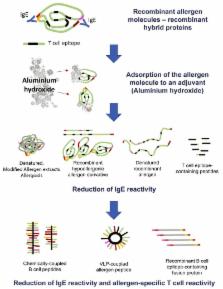
In the past, the development of more effective, safe, convenient, broadly applicable, and easy to manufacture vaccines for allergen-specific immunotherapy (AIT) has been limited by the poor quality of natural allergen extracts. Progress made in the field of molecular allergen characterization has now made it possible to produce defined vaccines for AIT and eventually for preventive allergy vaccination based on recombinant DNA technology and synthetic peptide chemistry. Here we review the characteristics of recombinant and synthetic allergy vaccines that have reached clinical evaluation and discuss how molecular vaccine approaches can make AIT more safe and effective and thus more convenient. Furthermore, we discuss how new technologies can facilitate the reproducible manufacturing of vaccines of pharmaceutical grade for inhalant, food, and venom allergens. Allergy vaccines in clinical trials based on recombinant allergens, recombinant allergen derivatives, and synthetic peptides allow us to target selectively different immune mechanisms, and certain of those show features that might make them applicable not only for therapeutic but also for prophylactic vaccination.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Long-term clinical efficacy of grass-pollen immunotherapy.
- Record: found
- Abstract: found
- Article: not found
Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study.
- Record: found
- Abstract: found
- Article: not found
