- Record: found
- Abstract: found
- Article: found
Function, Regulation and Biological Roles of PI3Kγ Variants
Read this article at
Abstract
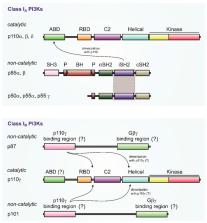
Phosphatidylinositide 3-kinase (PI3K) γ is the only class IB PI3K member playing significant roles in the G-protein-dependent regulation of cell signaling in health and disease. Originally found in the immune system, increasing evidence suggest a wide array of functions in the whole organism. PI3Kγ occur as two different heterodimeric variants: PI3Kγ (p87) and PI3Kγ (p101), which share the same p110γ catalytic subunit but differ in their associated non-catalytic subunit. Here we concentrate on specific PI3Kγ features including its regulation and biological functions. In particular, the roles of its non-catalytic subunits serving as the main regulators determining specificity of class IB PI3Kγ enzymes are highlighted.
Related collections
Most cited references182
- Record: found
- Abstract: found
- Article: not found
Phosphatidylinositol-3-OH kinase as a direct target of Ras.
- Record: found
- Abstract: found
- Article: not found
Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine.
- Record: found
- Abstract: found
- Article: not found