- Record: found
- Abstract: found
- Article: found
Rapid effects of extrafine beclomethasone dipropionate/formoterol fixed combination inhaler on airway inflammation and bronchoconstriction in asthma: a randomised controlled trial
Read this article at
Abstract
Background
The dose-dependent anti-inflammatory effects of a recent fixed combination of extrafine beclomethasone dipropionate/formoterol (BDP/F) were investigated using non-invasive markers of inflammation, exhaled nitric oxide (NO) and adenosine monophosphate (AMP) provocative challenge. The aim was to assess the onset of the anti-inflammatory action of low and high doses and evaluate the suitability of non-invasive assessments to demonstrate dose response.
Methods
Steroid naïve adult out-patients with mild asthma, sensitive to AMP with baseline exhaled NO > 25 parts per billion entered a double-blind, placebo-controlled, 3-way, cross-over study. Patients were randomised to low dose (1 actuation) or high dose (4 actuations) extrafine BDP/F 100/6 μg, or placebo administered twice daily on Days 1 and 2 and once in the morning on Day 3 of each period. Exhaled NO was measured pre-dose on Day 1, then 2 and 4 hours post-administration on Day 3. The AMP challenge was performed 4 hours post-administration on Day 3 and forced expiratory volume in 1 second (FEV 1, L) was measured from 0 to 4 hours post-dose on Day 1. Endpoints were NO at 2 and 4 hours, AMP challenge at 4 hours after the fifth dose on Day 3 and FEV 1 area under the curve from 0 to 4 h post-dose on Day 1. Analysis of covariance was performed for NO and FEV 1 and analysis of variance for AMP challenge.
Results
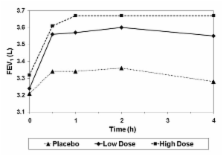
Eighteen patients were randomised and completed the study. Exhaled NO was significantly lower for both doses of extrafine BDP/F versus placebo at 2 and 4 hours (high dose LS mean difference: -22.5 ppb, p < 0.0001 and -20.5 ppb, p < 0.0001; low dose: -14.1 ppb, p = 0.0006 and -12.1 ppb, p = 0.0043) with a significant dose response (p = 0.0342 and p = 0.0423). Likewise, AMP challenge revealed statistically significant differences between both doses of extrafine BDP/F and placebo (high dose LS mean difference: 4.8 mg/mL, p < 0.0001; low dose: 3.7 mg/mL, p < 0.0001), and a significant dose response (p = 0.0185). FEV 1 was significantly improved versus placebo for both doses (high dose LS mean difference: 0.2 L, p = 0.0001; low dose: 0.2 L p = 0.0001), but without a significant dose response.
Related collections
Most cited references23
- Record: found
- Abstract: not found
- Article: not found
Asthma. From bronchoconstriction to airways inflammation and remodeling.
- Record: found
- Abstract: not found
- Article: not found
Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999.
- Record: found
- Abstract: found
- Article: not found