- Record: found
- Abstract: found
- Article: found
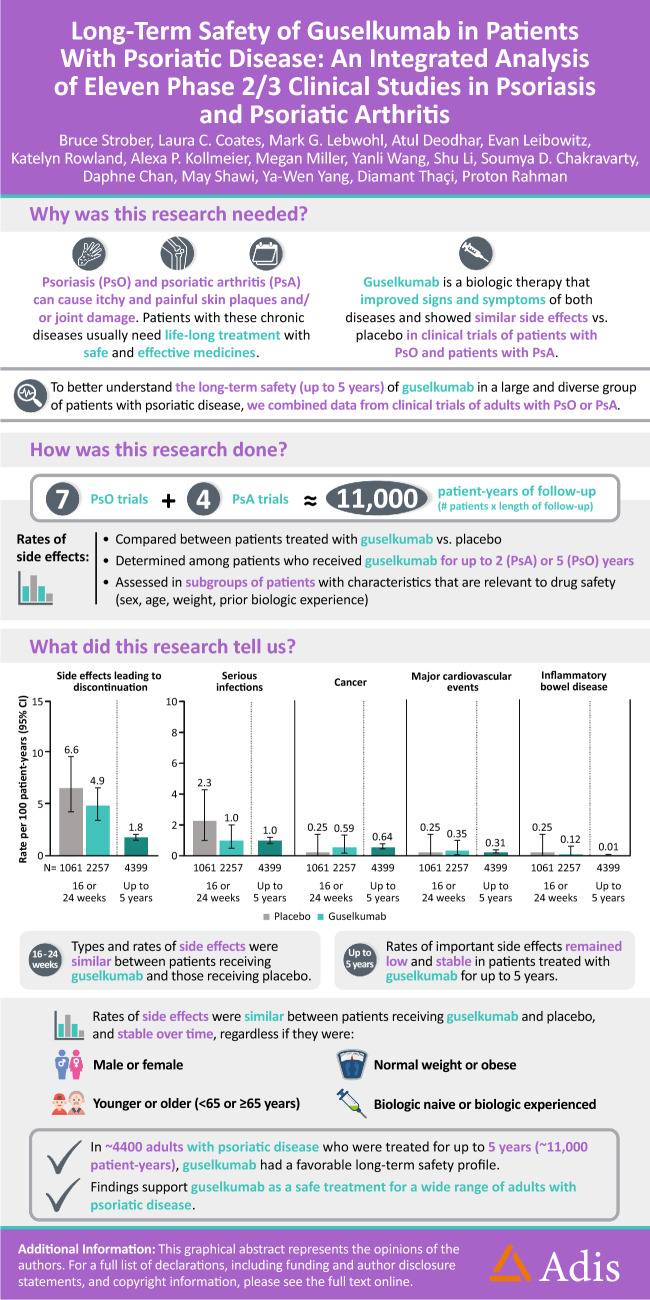
Long-Term Safety of Guselkumab in Patients with Psoriatic Disease: An Integrated Analysis of Eleven Phase II/III Clinical Studies in Psoriasis and Psoriatic Arthritis

Read this article at
Abstract
Introduction
The benefit/risk profiles of biologics can be affected by comorbidities, certain demographic characteristics, and concomitant medications; therefore, it is important to evaluate the long-term safety profiles of biologics across broad patient populations. Guselkumab was well tolerated and efficacious across individual pivotal clinical studies in adults with moderate-to-severe psoriasis and/or active psoriatic arthritis (PsA).
Objectives
The objective of the current analysis was to evaluate guselkumab safety in a large population of patients with psoriatic disease by pooling adverse event (AE) data from 11 phase II/III studies (seven in psoriasis; four in PsA).
Methods
Guselkumab was generally administered as 100 mg subcutaneous injections at Week 0, Week 4, then every 8 weeks (Q8W) in psoriasis studies and at Week 0, Week 4, then every 4 weeks (Q4W) or Q8W in PsA studies. Safety data were summarized for the placebo-controlled period (Weeks 0–16 in psoriasis; Weeks 0–24 in PsA) and through the end of the reporting period (up to 5 years in psoriasis; up to 2 years in PsA). Using the integrated data, incidence rates of key AEs were determined post hoc, adjusted for duration of follow-up, and reported per 100 patient-years (PYs). AE rates were also determined in subgroups of patients defined by sex, age, body mass index (BMI), and prior biologic use.
Results
During the placebo-controlled period, 1061 patients received placebo (395 PYs) and 2257 received guselkumab (856 PYs). Through the end of the reporting period, 4399 guselkumab-treated patients contributed 10,787 PYs of follow-up. During the placebo-controlled period, in the guselkumab and placebo groups, respectively, rates of AEs were 281 versus 272/100 PYs, and infections were 76.0 versus 72.2/100 PYs. Rates of serious AEs (5.6 vs. 7.8/100 PYs), AEs leading to discontinuation (4.9 vs. 6.6/100 PYs), serious infections (1.0 vs. 2.3/100 PYs), malignancy (0.59 vs. 0.25 patients/100 PYs), and major adverse cardiovascular events (MACE; 0.35 vs. 0.25/100 PYs) were low and comparable between guselkumab and placebo. Among guselkumab-treated patients, safety event rates through the end of the reporting period were numerically lower than or comparable with rates observed during the placebo-controlled period: AEs, 164/100 PYs; infections, 61.2/100 PYs; serious AEs, 5.4/100 PYs; AEs leading to discontinuation, 1.8/100 PYs; serious infections, 1.0/100 PYs; malignancy, 0.6/100 PYs; and MACE, 0.3/100 PYs. No AEs of Crohn’s disease, ulcerative colitis, or active tuberculosis were reported among guselkumab-treated patients. In the psoriasis studies, no opportunistic infections were reported among guselkumab-treated patients. Three AEs of opportunistic infections were reported in guselkumab-treated patients with PsA (0.14/100 PYs; all after Week 52 in DISCOVER-2). AE rates were largely consistent across subgroups of guselkumab-treated patients defined by sex, age, BMI, and prior biologic use.
Conclusions
In this analysis of 4399 guselkumab-treated patients with psoriatic disease followed for 10,787 PYs, guselkumab had a favorable AE profile. AE rates were similar between guselkumab- and placebo-treated patients and were consistent throughout long-term guselkumab treatment and across broad subgroups of patients with psoriatic disease.
Related collections
Most cited references83
- Record: found
- Abstract: found
- Article: not found
Classification criteria for psoriatic arthritis: development of new criteria from a large international study.
- Record: found
- Abstract: found
- Article: not found
Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis
- Record: found
- Abstract: found
- Article: not found