- Record: found
- Abstract: found
- Article: found
Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment
Read this article at
Abstract
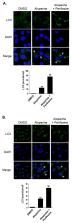
Aloperine, an alkaloid isolated from Sophora alopecuroides, exhibits multiple pharmacological activities including anti-inflammatory, antioxidant, antiallergic, antinociceptive, antipathogenic, and antitumor effects. Furthermore, it exerts protective effects against renal and neuronal injuries. Several studies have reported antitumor effects of aloperine against various human cancers, including multiple myeloma; colon, breast, and prostate cancers; and osteosarcoma. Cell cycle arrest, apoptosis induction, and tumorigenesis suppression have been demonstrated following aloperine treatment. In a previous study, we demonstrated antitumor effects of aloperine on human thyroid cancer cells through anti-tumorigenesis and caspase-dependent apoptosis induction via the Akt signaling pathway. In the present study, we demonstrated the modulation of the autophagy mechanism following the incubation of multidrug-resistant papillary and anaplastic human thyroid cancer cells with aloperine; we also illustrate the underlying mechanisms, including AMPK, Erk, JNK, p38, and Akt signaling pathways. Further investigation revealed the involvement of the Akt signaling pathway in aloperine-modulated autophagy in human thyroid cancer cells. These results indicate a previously unappreciated function of aloperine in autophagy modulation in human thyroid cancer cells.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Autophagy in acute kidney injury.
- Record: found
- Abstract: found
- Article: not found