- Record: found
- Abstract: found
- Article: found
HIF-2 α and Oct4 have synergistic effects on survival and myocardial repair of very small embryonic-like mesenchymal stem cells in infarcted hearts
Read this article at
Abstract
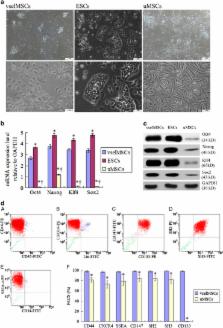
Poor cell survival and limited functional benefits have restricted mesenchymal stem cell (MSC) efficacy for treating myocardial infarction (MI), suggesting that a better understanding of stem cell biology is needed. The transcription factor HIF-2 α is an essential regulator of the transcriptional response to hypoxia, which can interact with embryonic stem cells (ESCs) transcription factor Oct4 and modulate its signaling. Here, we obtained very small embryonic-like mesenchymal stem cells (vselMSCs) from MI patients, which possessed the very small embryonic-like stem cells' (VSELs) morphology as well as ESCs' pluripotency. Using microarray analysis, we compared HIF-2 α-regulated gene profiles in vselMSCs with ESC profiles and determined that HIF-2 α coexpressed Oct4 in vselMSCs similarly to ESCs. However, this coexpression was absent in unpurified MSCs (uMSCs). Under hypoxic condition, vselMSCs exhibited stronger survival, proliferation and differentiation than uMSCs. Transplantation of vselMSCs caused greater improvement in cardiac function and heart remodeling in the infarcted rats. We further demonstrated that HIF-2 α and Oct4 jointly regulate their relative downstream gene expressions, including Bcl2 and Survivin; the important pluripotent markers Nanog, Klf4, and Sox2; and Ang-1, bFGF, and VEGF, promoting angiogenesis and engraftment. Importantly, these effects were generally magnified by upregulation of HIF-2 α and Oct4 induced by HIF-2 α or Oct4 overexpression, and the greatest improvements were elicited after co-overexpressing HIF-2 α and Oct4; overexpressing one transcription factor while silencing the other canceled this increase, and HIF-2 α or Oct4 silencing abolished these effects. Together, these findings demonstrated that HIF-2 α in vselMSCs cooperated with Oct4 in survival and function. The identification of the cooperation between HIF-2 α and Oct4 will lead to deeper characterization of the downstream targets of this interaction in vselMSCs and will have novel pathophysiological implications for the repair of infarcted myocardium.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease.
- Record: found
- Abstract: found
- Article: not found
HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth.
- Record: found
- Abstract: found
- Article: not found