- Record: found
- Abstract: found
- Article: not found
Dynamic Adipocyte Phosphoproteome Reveals that Akt Directly Regulates mTORC2
Read this article at
Summary
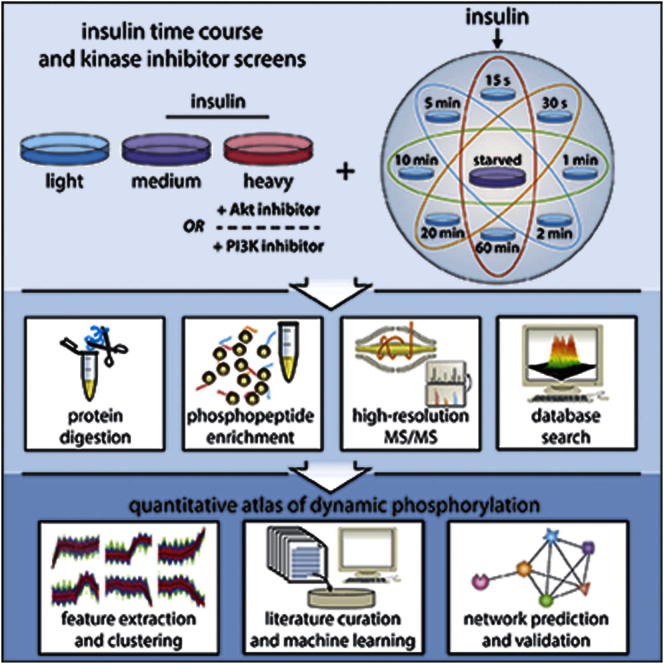
A major challenge of the post-genomics era is to define the connectivity of protein phosphorylation networks. Here, we quantitatively delineate the insulin signaling network in adipocytes by high-resolution mass spectrometry-based proteomics. These data reveal the complexity of intracellular protein phosphorylation. We identified 37,248 phosphorylation sites on 5,705 proteins in this single-cell type, with approximately 15% responding to insulin. We integrated these large-scale phosphoproteomics data using a machine learning approach to predict physiological substrates of several diverse insulin-regulated kinases. This led to the identification of an Akt substrate, SIN1, a core component of the mTORC2 complex. The phosphorylation of SIN1 by Akt was found to regulate mTORC2 activity in response to growth factors, revealing topological insights into the Akt/mTOR signaling network. The dynamic phosphoproteome described here contains numerous phosphorylation sites on proteins involved in diverse molecular functions and should serve as a useful functional resource for cell biologists.